Stop Germs at the Door
Our shoe sanitizing machine (UVZone) is a powerful, eco-friendly, and effective shoe disinfection solution utilized across clean manufacturing including Biotech, Pharmaceutical, Optical and More.
Our soltution is a better alternative to
Sanitizing Footbath Mats
Eliminate the variability and mess of footbath mats with a controlled entry solution.
Heres why sanitizing footbath mats don't work properly:
- Foot bath mats and footbath mats rely on chemicals that quickly become diluted, contaminated, and inconsistent under real foot traffic.
- Traditional sanitizing mats allow pathogens to spread as shoes track liquid and debris beyond the entry point.
- Footbath mats require constant monitoring, refilling, and disposal to remain effective—often failing between maintenance cycles.
- Low maintenance and long-term cost efficiency
- Ideal for healthcare, cleanrooms, food processing, and more

Finally...
A Shoe Sanitizing Machine That Actually Works
We call it the UVZone
Control the Spread of Germs with UVZone® Shoe Disinfection Technology
Actual Use Case:
Healthcare Sector: Gaylord Specialty Healthcare, Connecticut
Gaylord Specialty Healthcare became the first facility in New England to implement the UVZone® Shoe Sanitizing Station as part of its comprehensive infection control strategy during the COVID-19 pandemic. Recognizing that over 75% of individuals carry harmful pathogens on their shoes, the facility integrated UVZone® to eliminate up to 99.999% of pathogens, including MRSA, C. diff, E. coli, and Candida auris, in just 8 seconds. This proactive measure significantly enhanced the safety of both patients and staff by reducing the risk of pathogen transmission via footwear. Click here to verify the source of this use case.
Actual Use Case:
Public Safety Sector: Fire and Police Departments Nationwide
Green Science Solutions has deployed UVZone® Shoe Sanitizing Stations across various fire and police departments throughout the United States. These installations aim to mitigate the spread of viruses and bacteria, ensuring that first responders do not inadvertently carry harmful pathogens into their facilities. The rapid disinfection process, eliminating up to 99.999% of pathogens, provides an added layer of protection for personnel who are frequently exposed to diverse environments. Click here to verify the source of this use case.
Actual Use Case:
Agriculture Sector : Swine Production Facilities
Who it's for
UVZone® is built for facilities where cleanliness starts at the door.
From hospitals and cleanrooms to food processors, labs, and manufacturing plants — if your operation demands contamination control at entry points, UVZone® fits right in.
-
Hospitals & Healthcare Facilities – Infection control begins at the floor.
-
Clean Manufacturing – Semiconductor, aerospace, and pharma plants with strict particulate limits.
-
Laboratories & Biotech Facilities – Where sterile environments are critical.
-
Food Processing Plants – To prevent cross-contamination from street to line.
-
Pharmaceutical Manufacturing – GMP-compliant clean zones require strict pathogen control.
Deeper Dive:
In clean manufacturing environments, where precision and contamination control are paramount, PathO₃Gen Solutions’ UVZone® Shoe Sanitizing Stations offer a critical line of defense. Utilizing a patented combination of UV-C light and ozone, these stations effectively eliminate up to 99.9993% of harmful pathogens from footwear in as little as 6 to 10 seconds. This rapid disinfection process is essential in preventing the introduction of contaminants into sensitive production areas, thereby safeguarding product integrity and ensuring compliance with stringent industry standards.
The UVZone® system is designed for seamless integration into existing facility workflows. Its automated operation requires no additional staffing, and the absence of chemicals means there’s no risk of residue or corrosion on flooring surfaces. With features like weight-activated sensors and customizable cycle times, the stations provide consistent and reliable performance. By placing these units at key entry points, clean manufacturing facilities can significantly reduce the risk of contamination, enhance overall biosafety, and maintain the high-quality standards their operations demand.
What does it do?
UVZone® is built for facilities where cleanliness starts at the door.
From hospitals and cleanrooms to food processors, labs, and manufacturing plants — if your operation demands contamination control at entry points, UVZone® fits right in.
-
Eliminates up to 99.9993% of pathogens – Uses UV-C light and ozone to disinfect footwear quickly and effectively.
-
Disinfects in 6 to 10 seconds – Rapid cycle time keeps entry points moving without delay.
-
Fully automated, touch-free operation – Weight-activated sensors ensure consistent, hands-free use.
-
No chemicals, no mess – Safe for all flooring types and doesn’t introduce residue into clean environments.
-
Supports ISO and GMP compliance – Reinforces clean protocols in high-standard manufacturing zones.
Deeper Dive:
UVZone® Shoe Sanitizing Stations combine UV-C light and ozone to actively kill up to 99.9993% of pathogens on the soles of shoes within seconds. This dual-disinfection method targets one of the most overlooked contamination vectors—footwear—right at the point of entry. The system works without chemicals, liquids, or wipes, ensuring zero residue and no risk to sensitive production flooring.
Designed for seamless integration into clean manufacturing workflows, UVZone® operates hands-free with no staff oversight. Its intelligent sensors activate instantly when stepped on, and customizable cycle durations ensure consistent disinfection for every user. The result is a safer, more compliant environment that protects equipment, products, and personnel from harmful microbial intrusion.
When to use it
UVZone® is built for facilities where cleanliness starts at the door.
From hospitals and cleanrooms to food processors, labs, and manufacturing plants — if your operation demands contamination control at entry points, UVZone® fits right in.
-
Before entering clean zones – Stops contaminants at the door before workers cross into controlled environments.
-
At shift changes – Ensures new personnel begin their work free of microbial transfer from previous locations.
-
After breaks or off-floor activity – Maintains cleanroom standards when employees re-enter production areas.
-
In tandem with gowning/PPE protocols – Complements handwashing, boot covers, and full-body suits without slowing workflow.
-
When meeting HACCP, GMP, or ISO requirements – Helps achieve and document compliance with safety and quality standards.
Deeper Dive:
Use UVZone® right where contamination risks begin—before anyone sets foot in critical manufacturing zones. The system is ideal at entrances to cleanrooms, processing lines, and production floors, especially in operations where even small amounts of microbial contamination can compromise product integrity or halt output. It’s also essential after breaks, warehouse access, or any off-floor movement, ensuring workers return to the line with sanitized footwear.
UVZone® supports industry-leading safety programs including HACCP, GMP, and ISO 14644 by reinforcing barrier protocols at the point of entry. Unlike manual systems or chemical footbaths, it delivers consistent, measurable results with no cleanup and no training required. It’s a passive but powerful addition to your compliance strategy—automated, repeatable, and proven.
Where to use it
UVZone® is built for facilities where cleanliness starts at the door.
From hospitals and cleanrooms to food processors, labs, and manufacturing plants — if your operation demands contamination control at entry points, UVZone® fits right in.
-
Cleanroom entryways – Protect sensitive environments where airborne or surface-based contamination can compromise production.
-
Gowning rooms and anterooms – Reinforce PPE protocols by sanitizing footwear before entry into sterile zones.
-
Main employee entrances – Install at primary access points to stop contaminants before workers step onto the floor.
-
High-traffic access corridors – Deploy at crossover areas between warehouse, packaging, and processing spaces.
-
Critical manufacturing thresholds – Place at transitions between general and clean manufacturing zones to maintain compliance integrity.
Deeper Dive:
UVZone® should be installed precisely where the risk of introducing contaminants is highest—at the threshold of clean-controlled spaces. Whether you’re operating a pharmaceutical facility, clean electronics assembly line, or food-grade production floor, footwear sanitation at the point of entry is one of the most effective contamination control measures. High-traffic areas, including staff entrances, gowning rooms, and crossover zones between departments, are ideal install locations.
By placing UVZone® at these strategic transition points, you create an invisible barrier that reinforces your facility’s hygiene protocols and regulatory compliance. This includes support for ISO-certified cleanrooms, FDA-inspected environments, and HACCP-driven food production lines. It works as a frontline defense in any facility where foot traffic meets microbiological sensitivity.
How to purchase
UVZone® is built for facilities where cleanliness starts at the door.
From hospitals and cleanrooms to food processors, labs, and manufacturing plants — if your operation demands contamination control at entry points, UVZone® fits right in.
-
We don’t sell this in a generic online store for a reason – Every facility has unique compliance needs, traffic patterns, and layout considerations.
-
Talk to a live human who understands your industry – Our team will walk you through how UVZone® fits into your workflow and safety protocols.
-
Custom recommendations, not cookie-cutter kits – From entry placement to power requirements, we tailor the solution to your facility.
-
Easy procurement and documentation support – We help with specs, quotes, and any compliance paperwork you need.
-
Support doesn’t end at purchase – You’ll have access to real techs, not just a tracking number.
Deeper Dive:
UVZone® is a facility-level solution—not a shelf product. To make sure it’s right for your operation, we start with a conversation. Our team is made up of real people who understand clean manufacturing, healthcare, food safety, and biotech protocols. We’ll learn how your facility runs, how people move through it, and how best to place and integrate the UVZone® system.
When you reach out, we’ll match you with a product expert—not a chatbot or a web store. We’ll provide layout suggestions, budget estimates, and answer every question you have about power, installation, and ongoing use. Your industry is too important for guesswork—and so is your contamination control strategy.
Shoe Sanitizing Station
The UVZone® Shoe Sanitizing Station offers an innovative solution to enhance facility cleanliness by effectively eliminating harmful pathogens from footwear.
Here’s how it works:
1. Step on the footprints, the station will light up.
2. Wait a few seconds.
3. When you hear the beep, you can walk forward. Your shoes have been sanitized.

Minimizing Risk
Improving Compliance
To help improve our compliance with USP 797 and minimize the risk of pathogens contaminating our clean room, we added the PathO3Gen Solutions’ Shoe Sanitizing Station to our department’s action plan.
The stations are now part of our process that each employee uses prior to entering our clean room.
Our last Air and Surface samples were negative for any growth in both rooms for the first time ever. We will continue to utilize the PathO3Gen Solutions Shoe Sanitizing Station in our ante room because the more tools we have to minimize risk, the better.
Jeffrey Miley, Pharm. D., CPh.
Director of Pharmacy Services, AdventHealth
Combat Shoe-Borne Pathogens
Key Features & Functions
Works in ≤ 10 Seconds
Allowing you to seamlessly integrate the UVZone shoe sanitizing stations into your protocols without compromising efficacy
Can be set to 6, 8 or 10 second cycle times, depending on requirements
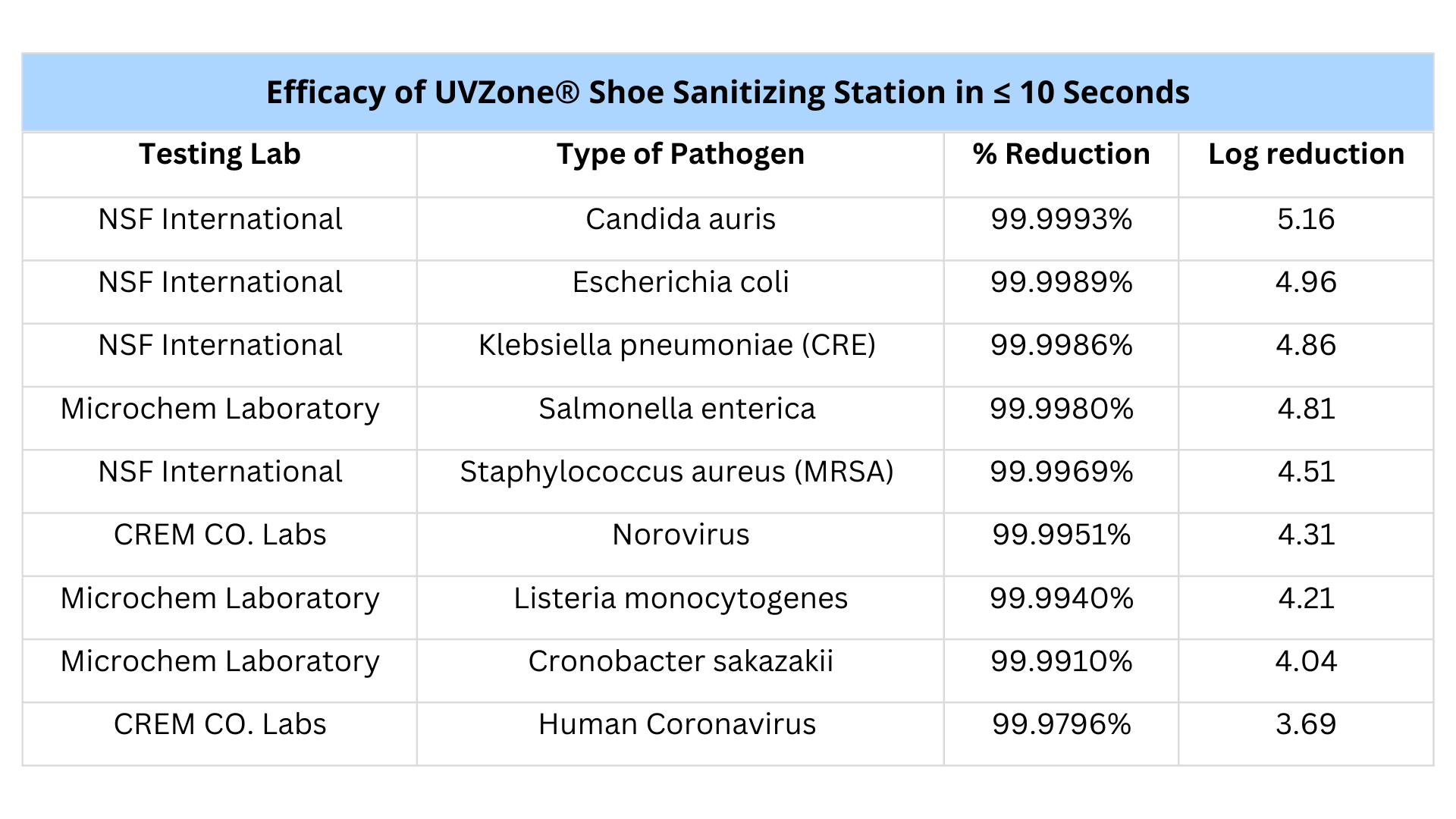
Up to 99.9993% kill rates
The shoe sanitizing station has a 3-5Log reduction with industry leading kill rates at only 8 seconds.
Third Party Tested + Certified
Independently tested and verified results for guaranteed efficacy and safety.
TÜD SÜV Certified | ISO 9001 Manufactured in USA
Plug & Play Functioning
Works right out of the box, plugging in to standard outlet, giving you peace of mind with minimal effort.
Requires No Additional Staff
Enhances Existing Biosecurity Measures
By protecting the perimeter of critical areas, UVZone reduces bioburden entering an area, therefore enhancing your existing measures’ efficacy
UVZone has been tested by world-renowned third party laboratories, including:

NSF International

CREM Co. Laboratories

Microchem Laboratory